Context
Recognising the importance of green hydrogen for India’s energy transition, the Indian cabinet approved the National Green Hydrogen Mission in January 2023. The mission aims to build 5 million metric tonnes (MMT) of green hydrogen production capacity by 20301 in order to make India a global hub for hydrogen production and exports. The mission estimates the investment requirements to be INR 8 lakh crores (approximately USD 100 billion). Renewable energy-based electricity, water, and electrolysers are the key components in the production of green hydrogen. Electrolysers are estimated to account for up to 36% of the levelised cost of green hydrogen.2
Principle behind the technology
During electrolysis, electricity is used to separate water into oxygen and hydrogen using electrolysers.3 Electrolysers can be classified into three levels: cell, stack, and system. The cell is the basic unit of an electrolyser and comprises of an electrode [cathode (-) and anode (+)], an electrolyte and a semi-permeable membrane. Splitting takes place in two partial processes at the electrodes. When electricity is passed through the electrolyser, the cathode makes hydrogen, and the anode makes oxygen. Stacks are groups of cells that are linked to each other, and the system level includes cooling, gas processing, and electricity conversion.3,4
Types of electrolysers
The principle behind electrolysis remains the same across technologies. However, the technologies differ on the basis of various physical, chemical and electrochemical aspects. At present, there are four main types of electrolysis technologies.
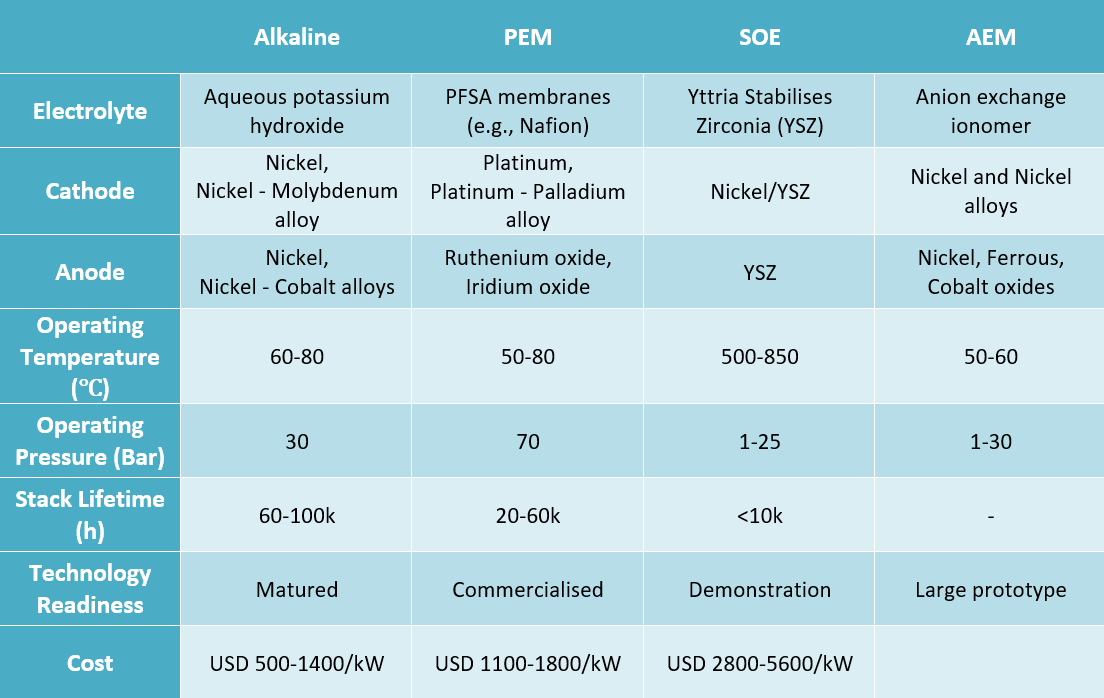
- Alkaline electrolysers Alkaline electrolysis is a mature and commercially available technology used primarily by the fertiliser and chlorine industries. It presently accounts for almost two-thirds of the global electrolyser capacity.5 It operates at a pressure of 30 bar and uses thick membranes and nickel-based electrodes.4,6 While its simple and relatively low-cost stack and system design makes it the cheapest electrolyser technology, its thick membranes reduce its efficiency to 70 - 80 per cent.7,8
- Proton exchange membrane (PEM) electrolysers - Despite being a young technology PEM electrolysers comprises one-fifth of the global capacity.5 PEMs operate at high pressure, due to the use of thin perfluorosulfonic acid (PFSA) membranes, which translates to an efficiency of 80 - 90 per cent4,6. PEM has a compact and simple design, and it has advantages in terms of operability with intermittent loads, i.e., fast response to varying renewable electricity7,8. However, the PFSA acidic environment makes it necessary to use gold and titanium plated electrodes and metals such as platinum, iridium, and ruthenium as catalysts, which increase its cost significantly.4
- Solid oxide electrolysis cell (SOEC) electrolysers - SOECs differ as they utilise heat to make hydrogen from steam and are best placed where there is a heat source available (nuclear or industrial facilities). They operate at high temperatures (500 - 850 ℃).7 SOECs have shown higher efficiency than other technologies; however, on the flip side, it is not suited to withstand load changes.8 The technology is still at demonstration level.5
- Anion exchange membrane (AEM) electrolysers - AEM electrolysers operate at significantly lower temperatures of 50 - 60 ℃ and a pressure range of 1 - 30 bar.6 They combine the less harsh conditions of alkaline electrolysers with the simplicity and high efficiency of PEM electrolysers.4 It is the latest technology, presently deployed at the large prototype level4, with only a few companies commercialising it.
Table 1: Specifications of 4 types of electrolysers
Source: CEEW-CEF analysis based on multiple sources5,6,7
Who should care?
· Green hydrogen producers
· Carbon-intensive industries
· Financial institutions
References
- [1] Ministry of New and Renewable Energy. 2022. “National Green Hydrogen Mission.” New Delhi, India: MNRE. https://mnre.gov.in/img/documents/uploads/file_f-1673581748609.pdf
- [2]CEEW-CEF. 2021. “Catalysing Green Hydrogen Growth in India.” CEEW/Centre for Energy Finance. https://www.ceew.in/cef/masterclass/analysis/catalysing-green-hydrogen-growth-in-india.
- [3] PtX Hub. 2021. “Water Electrolysis Explained”. https://ptx-hub.org/water-electrolysis-explained/.
- [4] IRENA. 2020. Green Hydrogen Cost Reduction: Scaling up Electrolysers to Meet the 1.5⁰C Climate Goal. Abu Dhabi: International Renewable Energy Agency.
- [5] IEA. 2022. “Electrolysers”. Paris: International Energy Agency. https://www.iea.org/reports/electrolyser
- [6] Lavacchi, Alessandro, Karel Bouzek, Jaromír Hnát, Stefan Loos, Christian Immanuel Müller, Thomas Weißgärber, Lars Röntzsch, and Jochen Meier-Haack. 2020. “Green Hydrogen from Anion Exchange Membrane Water Electrolysis: A Review of Recent Developments in Critical Materials and Operating Conditions.” Sustainable Energy and Fuels 4 (5): 2114–33. https://doi.org/10.1039/c9se01240k.
- [7] Kumar, Sanjay, and V. Himabindu. 2019. “Hydrogen Production by PEM Water Electrolysis – A Review.” Materials Science for Energy Technologies 2 (3): 442–54. https://doi.org/10.1016/j.mset.2019.03.002
- [8] Government of Scotland. 2022. “Assessment of Electrolysers: Report,”. http://www.gov.scot/publications/assessment-electrolysers-report/pages/1/.